High/Low-Temperature Sterilization and Decontamination in a Single Unit
Our RH-Pro line of sterilizers now feature 3 options: High Temperature and Low Temperature sterilization and N95 Mask Decontamination. These cycle options add significant flexibility to any practice’s sterilization process


RH-Pro9
The Industry’s Most Efficient Sterilizer
A proven advancement in “RapidCycle” sterilization, the RH-Pro9 sterilizer will raise your level of sterilization efficiency. Using CuttA proven advancement in “RapidCycle” sterilization, the RH-Pro9 sterilizer will raise your level of sterilization efficiency. Using Cutting-Edge HVHA (High-Velocity Hot Air) technology, the RH-Pro9 provides the fastest instrument turnaround of any FDA-Cleared sterilization technology. No steam or water means no lengthy drying cycle with longer instrument life free of corrosion.
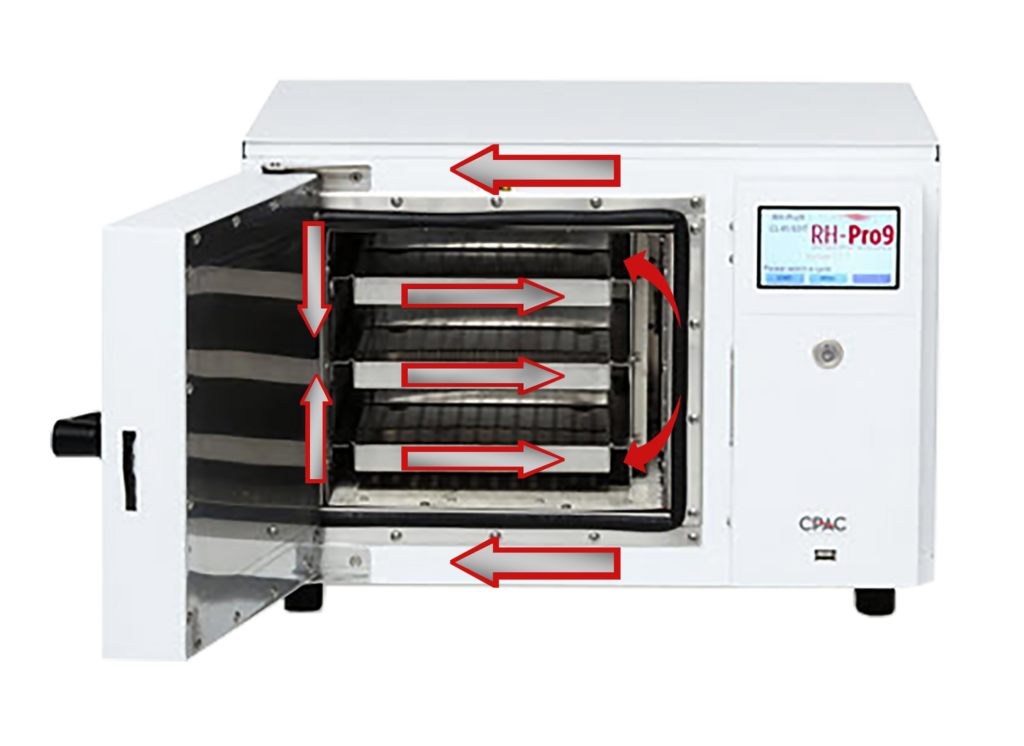
The Pro9 Features
- Rectangular chambers with more uniform capacity
- Easy and simple touch screen operation
- Non-corrosive waterless environment
- Quiet operation with NO emissions
- Uses 85% less energy than steam
Sterilization Times
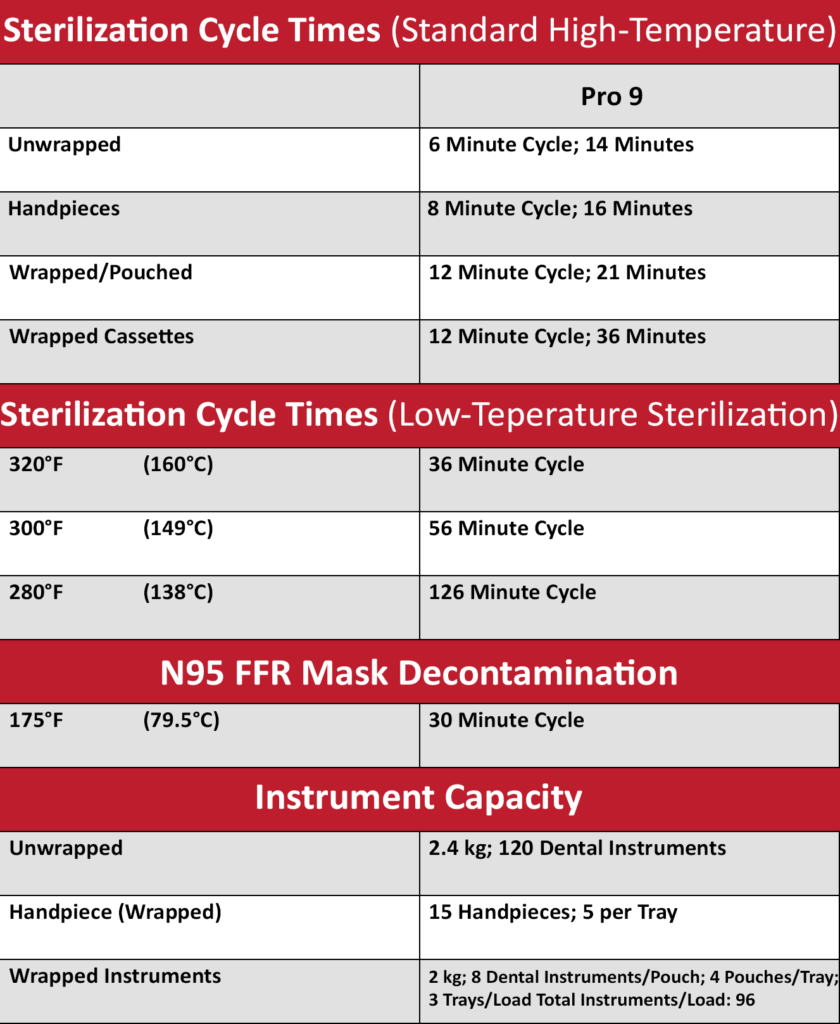
EXAMINE THE HVHA ADVANTAGE
- Waterless Sterilization
- Easy Touch Screen Operation
- Faster Processing
- More Energy-Efficient
- Less Maintenance & Down-Time
- Three Large Instrument Trays
- Fits Easily into Most Cabinets
RapidHeat vs. Steam
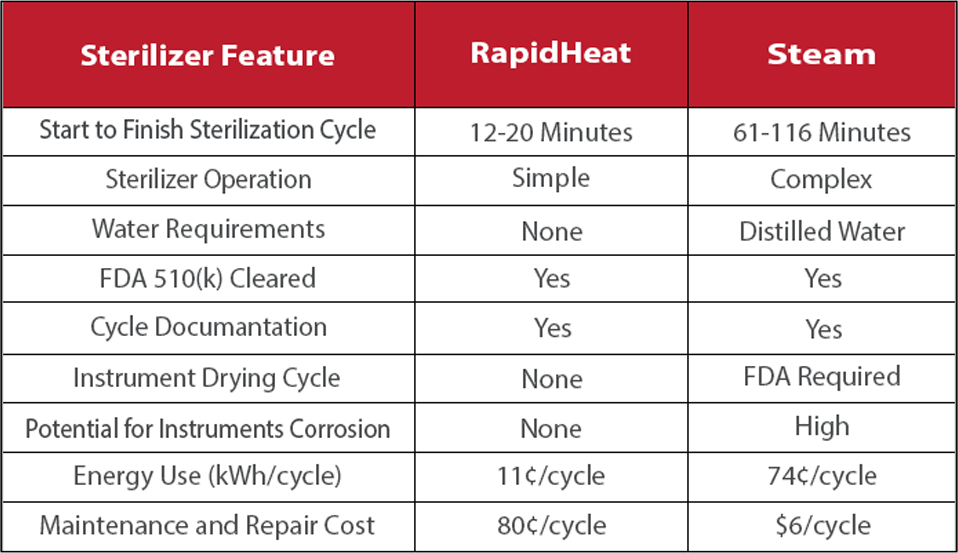
1) Start to Finish Cycle represents the time range for sterilizing unwrapped, pouched, and wrapped (cassette/tray) instruments from cold start to finish (includes dry cycle for steam).
2) Sterilizer Operation reflects the level of sterilizer preparation and management for instrument processing.
3) Potential for Instrument Corrosion is absent in the dry environment of a RapidHeat sterilizer and high for instruments in a steam environment.
4) Energy Use represents kilowatts of power used per hour when operating a sterilizer cycle. This study was conducted by the Rochester Institute
of Technology comparing RapidHeat to 2 popular tabletop steam sterilizers.
5) Maintenance and Repair Cost is based on actual reported costs and estimated over the useful life.
FAQs
Has the HVHA Sterilization Process Received FDA Clearance as
a Medical Product?
The FDA issued clearances for our RapidHeat sterilizers under K872643A and K881371 in 1987 and 1988, respectively. These units have ETL, UL, and applicable CE approvals. CPAC Equipment, Inc., the manufacturer of RapidHeat sterilizers, is an ISO 13485 certified manufacturing facility.
How Long has the HVHA Sterilization Technology been used in Dental and Healthcare Practices?
RapidHeat sterilizers have been in successful use for over 25 years in the dental and healthcare markets.
Can HVHA Sterilization Be Used Only on Metal Instrument?
Most of today’s instruments including handpieces and other instruments incorporating thermal plastic composite materials that are suitable for steam sterilization are also suitable for the higher temperatures of RapidHeat sterilizers with no negative effect on the instrument lifecycle. In recent years the creation of more heat-tolerant materials (e.g., heat-resistant fluoropolymers. silicones, and polycarbonates) and their replacement of heat-intolerant materials used in medical devices has reduced significantly the number of instruments that are intolerant to dry heat sterilization conditions.
Can the RapidHeat Sterilizer replace "Immediate-Use" Sterilization Practice?
RapidHeat sterilizers have pre-set sterilization cycles ranging from six minutes to twenty-one minutes depending on load configuration, packing configuration, and load size. These short sterilization cycle times and and no drying requirement makes HVHA technology an excellent choice to replace “Immediate-Use steam sterilization.” RapidHeat Sterilizer cycles do not fall under healthcare’s definition of “immediate-use” which is a standard term used for short-cutting the drying time needed in steam sterilization. An “Immediate-Use” steam sterilization cycle is used by staff when an instrument or instrument set has an emergency need to be turned around as quickly as possible. If there is improper drying, subsequent cooling will cause any moisture to condense and packaged instruments to remain wet, increasing the potential for instrument corrosion. Moisture degrades the ability of the packaging to maintain sterility. Failure to dry instruments after steam sterilization violates CDC’s recommendations that state “instrument packs should be allowed to dry inside the sterilizer chamber before removing and handling. Packs should not be touched until they are cool and dry because hot packs act as wicks, absorbing moisture, and hence, bacteria from hands.”
What is the Comparative Cost to Operate on the RH Sterilizer?
A Comparative Cost Analysis reveals a 50% operating cost savings over a comparable steam sterilizer. Exceptional savings are found in maintenance, utility, and instrument replacement costs. Operational cost can be defined by utility costs, sterilizer–required supply costs, and equipment maintenance cost.
Providing quality sterile processing and related products that enhance patient safety to dental and healthcare industries while reducing patient treatment costs for more than 25 years.
FULL 3 YEAR WARRANTY!

